Disinfection

Filtration Technology
4 October 2020
Aeration
6 October 2020
Disinfection
Virtually all regulations concerning water quality and treatment are aimed at the protection of public health through the elimination of waterborne disease. The primary means of accomplishing this is through disinfection of drinking water to inactivate bacteria, viruses and protozoa. Water disinfection is also very common in water treatment processes since the presence of bacteriological contamination may cause undesirable negative effects on plant equipment or to the treatment process itself. There are today several methods of water disinfection with the following as the prevailing.

Ultraviolet Radiation
Ultraviolet (UV) light produced from UV lamps has been shown to be an effective bactericide for both air and water. In disinfecting water, the quantity of radiation required is dependent on such factors as turbidity, color and dissolved iron salts, which adversely affect the penetration of ultraviolet energy through the water. For this reason, UV light is not satisfactory for disinfecting water with high turbidity. Cylindrical UV disinfection units with standard plumbing fittings have been designed for in-line installation in water lines. The intensity of the light should be checked frequently and the sleeve around the bulb periodically cleaned. Some units have an externally operated plunger-type apparatus for this purpose. A disadvantage of UV light is that it does not provide a disinfectant residual in the water as does chlorine. Thus, there is no barrier against recontamination of the water. For this reason, UV light is not approved by the Environment Protection Agency as a sole method of disinfection for public water systems. Another disadvantage is that a continuous source of electrical power is required to operate an ultraviolet light.

Ozone
While ozone is widely used for disinfection and oxidation in other parts of the world, its use is relatively new in Europe. Ozone is unstable at ambient temperatures and pressures and therefore must be generated on site and used quickly. It is generated by applying a high-energy electrical field to either pure oxygen or dried air. Disinfection takes place as ozone gas is added to flowing water. The half-life of ozone in water is fairly short, on the order of minutes, due to its decomposition back to oxygen. For this reason, a secondary disinfectant is required, as with UV light, so that a disinfectant residual can be maintained and measured throughout the distribution system. One advantage of ozone is that it forms few disinfectants by products such as trihalomethanes (THMs). Disadvantages are its relatively high cost and the complexity inherent in on site generation.
Chlorination
Chlorine is a disinfectant added to water to reduce or eliminate microorganisms, such as bacteria and viruses, which can be present in water supplies. Disinfecting our drinking water ensures it is free of the microorganisms that can cause serious and life-threatening diseases, such as cholera and typhoid fever. To this day, chlorine remains the most commonly used water disinfectant, and the disinfectant for which we have the most scientific information. Chlorine is added as part of the drinking water treatment process. However, chlorine also reacts with the organic matter, naturally present in water, such as decaying leaves. This chemical reaction forms a group of chemicals known as disinfection by-products. The most common of these by-products are trihalomethanes (THMs), which include chloroform. The amount of THMs found in drinking water depends on a number of things, including the season and the source of the water. For example, THM levels are generally lower in winter than in summer, because the amount of natural organic matter is lower and less chlorine is needed to disinfect at colder temperatures. THM levels are also low when wells or large lakes are the drinking water source, and higher when rivers or other surface waters are the source, because they generally contain more organic matter. Current scientific data shows that the benefits of chlorinating our water (less disease) are much greater than any health risks from THMs and other by-products.
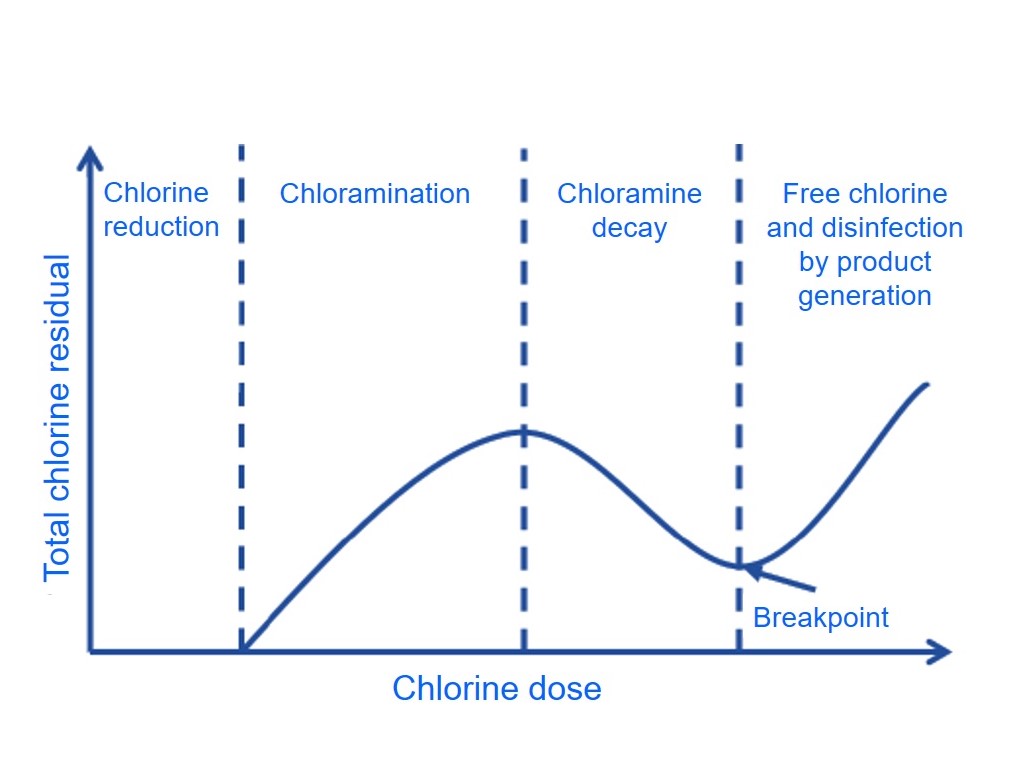
The Benefits of free chlorine as disinfection agent
Potent Germicide
Chlorine disinfectants can reduce the level of many disease-causing microorganisms in drinking water to almost immeasurable levels.
Taste and Odor Control
Chlorine disinfectants reduce many disagreeable tastes and odors. Chlorine oxidizes many naturally occurring substances such as foul-smelling algae secretions, sulphides and odors from decaying vegetation.
Biological Growth Control
Chlorine disinfectants eliminate slime bacteria, moulds and algae that commonly grow in water supply reservoirs, on the walls of water mains and in storage tanks.
Chemical Control
Chlorine disinfectants destroy hydrogen sulphide (which has a rotten egg odor) and remove ammonia and other nitrogenous compounds that have unpleasant tastes and hinder disinfection. They also help to remove iron and manganese from raw water.

